Clean in Place CIP training is designed for people employed across the food sectors who employ CIP systems and SIP processes as part of GMP, hygiene, and cleaning systems.
Course Cost: POA
About Clean-in-Place CIP Training
Food Safe’s CIP cycle training is designed for people employed across the food sectors who employ CIP and SIP cleaning processes as part of their cleaning, hygiene, and GMP systems.
CIP is an effective cleaning sequence that replaces manual cleaning used in processing lines and factories, where constant proper cleaning of interior surfaces such as process equipment is of utmost importance for product safety. The automated method of this cleaning cycle means that a more concentrated acid and caustic wash cycle can be used, which reduces energy consumption and the cleaning time of processing equipment, compared to being cleaned manually with cleaning chemicals at lower temperatures.
Our training further builds operator verification skills, on-job competency awareness around chemical exposure risk, and cross contamination as well as microbiology while improving the company’s CIP systems effectiveness.
Cleaning equipment effectively is a critical part of any business. Our training course is suitable for: all operators, coordinators, team leaders, supervisors, engineers, managers, and staff interested in further developing their cleaning efficiency skills.
Clean-In-Place (CIP) Training Level 3-4 Dairy Sector:
Food Safe collaborates with SSBs to offer training programmes. Registration includes an SSB training agreement and administration. This training as well as the New Zealand Certificate in Dairy Processing – Levels 3-5 is available on request.
We offer CIP training for all other food sectors including food and beverage, meat, seafood, poultry, and egg processing to name a few.
Clean in Place CIP Training Course Content
- An introduction to the CIP system and critical parameters around CIP equipment
- An introduction to best practice cleaning process and cleaning solutions
- An introduction to microbiology and an understanding of how CIP and SIP systems are used to prevent microbiological and bacterial growth and contamination of the product
- An overview of the different types of cleaning solution and sanitisers and how this relates to pH knowledge
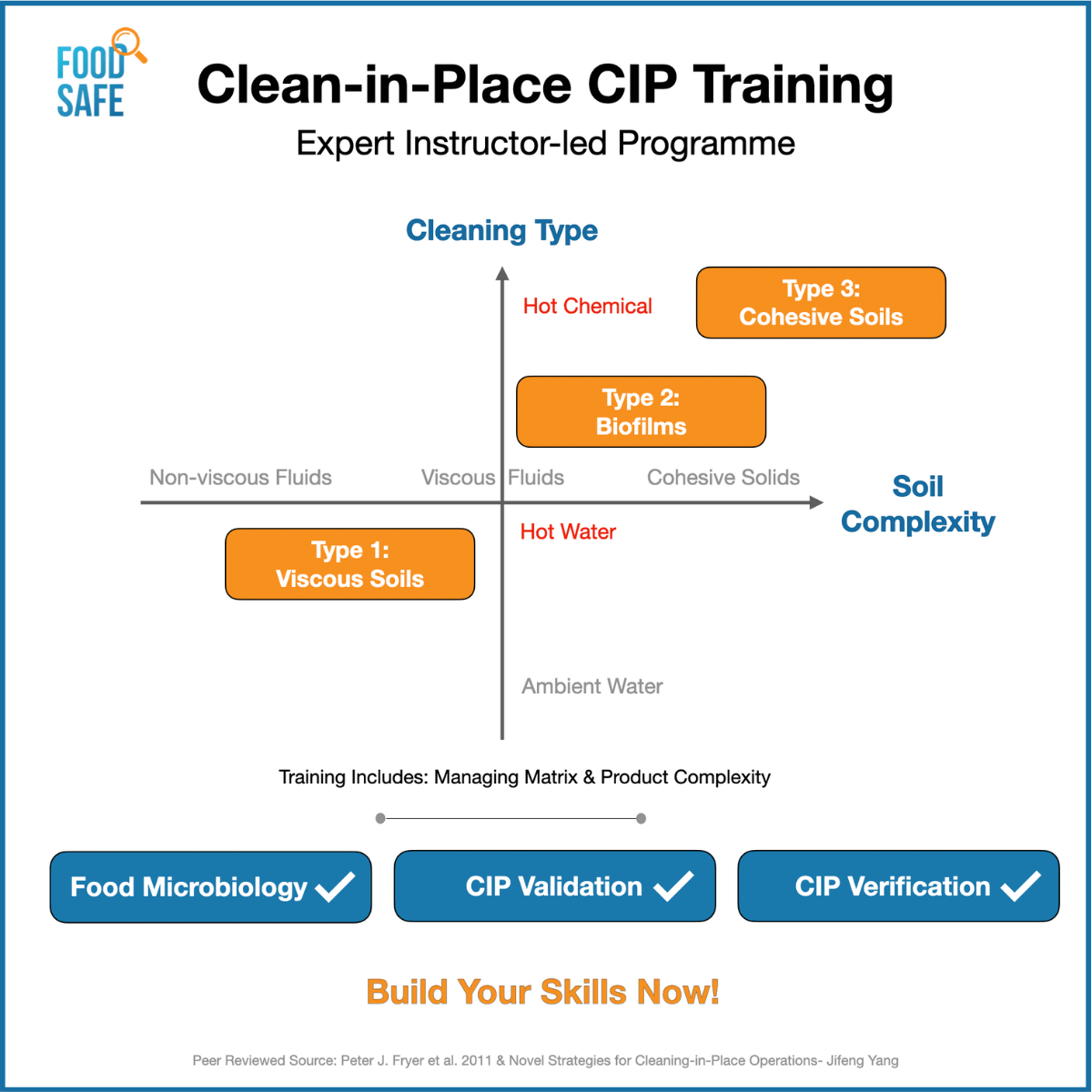
- An introduction to the right cleaning chemicals for the right soils
- Introduction to key components for CIP system to be effective (time, temperature, flow, chemical, concentration, and soil loads)
- Optimal timings for CIP; what are the points to keep in mind if the timing is not automated
- An introduction to titration checks and its role in CIP cycles
- How effective CIP is assessed – general basic CIP method checks
- Corrective and preventive actions
Delivered On-site or Live Online via Video Conference Globally
Auckland
Beijing
Dubai
Vancouver
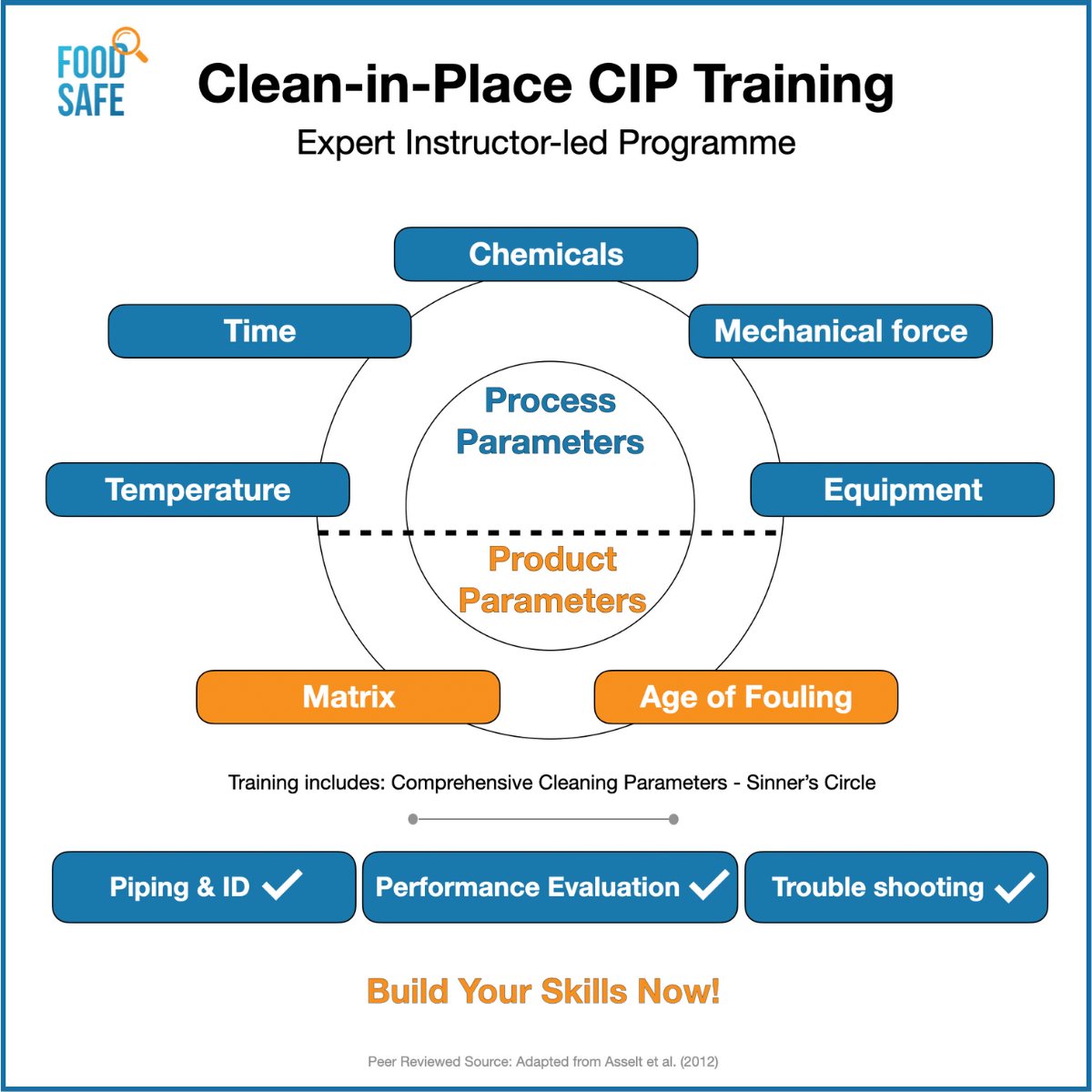
Some Useful Clean-in-Place Performance Checks:
General CIP Performance Evaluation Checks
Based on our experience and expertise, we have listed a few key points for your operations team below:
- CIP Performance checked by technically trained personnel? ☐
- Equipment checked after each cleaning cycle for cleanliness? ☐
- Water analysis of CIP makeup water done? ☐
- System operation reviewed as a team including management, supervisors, and operators? ☐
- Equipment operating properly and in good condition? ☐
- Cleaning chemical concentration checks are within Sanitation Standard Operating Procedure (SSOP) ranges? ☐
- At least one complete CIP cycle/program on a regular basis and documented? ☐
Spray CIP Performance Evaluation Checks
Here are a few main points for your operations team based on our experience and expertise, below:
- Appearance of the final burst rinse checked? ☐
- Is it clear or is it hazy/opaque?
*Normal rule is to flush until the discharge runs clear, if not clear then probably indicates that there is a problem.
- Is it clear or is it hazy/opaque?
- Check for valve pulsing (such as tank outlet valve and other air-operated valves inline? ☐
- Ensure both sides of the plunger and stem are getting cleaned?
- Wash and rinse cycles check temperatures checked? ☐
- Note: Use a portable digital temperature probe to confirm the accuracy of the recording chart.
Frequently Asked Questions – FAQ
What is Cleaning?
Cleaning is the complete removal of food soil using appropriate detergent chemicals under recommended conditions. Peer-reviewed source: University of Florida & FDA
What does Cleaning Methods mean?
Equipment can be categorized with regard to cleaning method as follows:
• Mechanical Cleaning. Often referred to as clean-in-place (CIP). Requires no disassembly or partial disassembly.
• Clean-out-of-Place (COP). Can be partially disassembled and cleaned in specialized COP pressure tanks.
• Manual Cleaning. Requires total disassembly for cleaning and inspection.
Peer-reviewed source: University of Florida & FDA
What does Sanitization mean?
Sanitization is referred to by different some different terminology below:
• Sterilize refers to the statistical destruction and removal of all living organisms.
• Disinfect refers to inanimate objects and the destruction of all vegetative cells (not spores).
General types of sanitization also include:
• Thermal Sanitization involves the use of hot water or steam for a specified temperature and contact time.
• Chemical Sanitization involves the use of an approved chemical sanitizer at a specified concentration and contact time.
Peer-reviewed source: University of Florida & FDA
What are some types of food soils that require cleaning?
Food soils may be broadly classified as follows:
- Soils soluble in water (sugars, some starches, most salts);
- Soils soluble in acid (limestone and most mineral deposits)
- Soils soluble in alkali (protein, fat emulsions)
Peer-reviewed source: University of Florida & FDA
What are fat-based soils?
Fat usually is present as an emulsion and can generally be rinsed away with hot water above the melting point.
More difficult fat and oil residues can be removed with alkaline detergents, which have good emulsifying or saponifying ingredients.
Peer-reviewed source: University of Florida & FDA
What are protein-based soils?
Protein-based soils are by far the most difficult soils to remove.
For example, casein (a major milk protein) is used for its adhesive properties in many glues and paints.
Food proteins range from more simple proteins, which are easy to remove, to more complex proteins, which are very difficult to remove.
Heat-denatured proteins can be extremely difficult.
Generally, a highly alkaline detergent with peptizing or dissolving properties is required to remove protein soils.
Wetting agents can also be used to increase the wettability and suspendability of proteins.
Protein films require alkaline cleaners that have hypochlorite in addition to wetting agents.
Peer-reviewed source: University of Florida & FDA
What are carbohydrate-based soils?
Simple sugars are readily soluble in warm water and are quite easily removed.
Starch residues, individually, are also easily removed with mild detergents.
Starches associated with proteins or fats can usually be easily removed by highly alkaline detergents.
Peer-reviewed source: University of Florida & FDA
What are mineral salt-based soils?
Mineral salts can be either relatively easy to remove or be highly troublesome deposits or films. Calcium and magnesium are involved in some of the most difficult minerals films.
Under conditions involving heat and alkaline pH, calcium and magnesium can combine with bicarbonates to form highly insoluble complexes.
Other difficult deposits contain iron or manganese.
Salt films can also cause corrosion of some surfaces.
Difficult salt films require an acid cleaner (especially organic acids that form complexes with these salts) for removal.
Sequestering agents such as phosphates or chelating agents are often used in detergents for salt film removal.
Peer-reviewed source: University of Florida & FDA
What are Microbiological Films?
Under certain conditions, microorganisms (bacteria, yeasts, and molds) can form invisible films (biofilms) on surfaces.
Biofilms can be difficult to remove and usually require cleaners as well as sanitizers with strong oxidizing properties.
Peer-reviewed source: University of Florida & FDA
Clean-In-Place Resources
Cleaning-in-Place: Dairy, Food, and Beverage Operations
Equipment Cleaning & Sanitizing in Food Processing
FDA Food Microbiology Guide
Current & Emergent Biofilm Control Strategies
FDA – Microbiological Quality of Processed Food
FSANZ – Microbiological Criteria for Food
About Food Safe
Food Safe Ltd is Accredited by the New Zealand Government + is a Category 1 NZQA-Registered PTE. Training complies with the Food Safety Bylaws and Verifier Audits right across New Zealand.
Our food safety training, quality assurance, and auditing services are trusted by both well-known New Zealand and Global food companies and heaps of small teams too!
Food Safe’s advisory committee includes leading experts, quality and compliance managers, and governance experts. For even more information about Food Safe and the companies we work with, click here
Why Choose us for your Training?
Our training:
- Complements compliance requirements
- Is simplified and visual, and supportive of implementing learning back on-job
- Is delivered by a trained ISO 9001 & 22000 lead auditor
- Is delivered by a trainer with first-hand knowledge and experience in high compliance process lines where Food Safe also operates, such as process plants in the meat and dairy products sectors

Here’s how we collaborate with companies
120
827
100
HOW IT WORKS
Companies we work with






























































